Anti-Urolithiatic and Diuretic Activities of Ethyl Acetate Fraction of Grewia flavescens Roots
AAPS Science360. Bhavsar G. 05/05/25; 4157504; M1130-02-06
Topic: rat
CLICK HERE TO LOGIN
RESERVED CONTENT
RESERVED CONTENT
This content is accessible by AAPS members and NBC2025 Registered participants (Use the “Participant Access” button at the top).
Use interactive features below when available.
Use interactive features below when available.
Abstract/Article
Discussion Forum (0)
Purpose: Urolithiasis is the most widespread urinary system disease that suffered globally. It formed due to deposition of acid salt in concentrated urine, which can cause infection, blockage, and damage to tissue of urinary system. Although the various conventional therapies are available for the urolithiasis, but side effects of the drugs cannot be avoided, application of extracorporeal lithotripsy, percutaneous nephrolithotomy and other minimally invasive techniques have made the treatment of urolithiasis more efficient in reducing their morbidity. In India, many herbal formulations are in use for the treatment of urolithiasis, known for its unquestionable efficacy and safety. Roots of Grewia flavescens used for treatment of urolithiasis. However, till date there is no scientific evaluation report of the above plant to be effective in treating urolithiasis. So, intends of present study to establish scientific rationality of Ethyl Acetate Fraction of Grewia flavescens (EAFGF) roots using in vitro study performed including nucleation assay, Calcium oxalate crystal growth and crystal aggregation assay, crystal dissolution, and Madin Darby Canine Kidney (MDCK) cell line study, while in vivo study performed using sodium oxalate induced urolithiasis model for preventive study and ethylene glycol induced model for curative study.
Methods: For in vitro study, calcium oxalate crystals were prepared using the fresh solution of sodium oxalate and calcium chloride. Crystal nucleation and crystal growth assay were detected in UV spectrometry at 620 nm and 214 nm, respectively. Crystal aggregation assay was measured using turbidity meter. In a small test tube calcium oxalate crystal weight was measured before and after completion of study, to determine its dissolution. All study performed at varying ranges of EAFGF (200 to 2000 µg/ml). Cell viability test and Lactate Dehydrogenase Level (LDH) release after exposure of oxalate with or without EAFGF (concentration range 0.05 to 50 µg/ml) performed in MDCK Cell line. The identification and quantification of quercetin, rutin, catechin, and gallic acid, in EAFGF extract was performed using HPTLC and validated HPLC method, respectively.
For In vivo study, diuretic activity of EAFGF was performed using diuretic model in wistar albino rats. Sodium oxalate model and ethylene glycol models were used for urolithitic activity. 70 mg/kg sodium oxalate was given intraperitoneally for 7 days to induce the kidney stone in rats. 0.75 % v/v Ethylene Glycol was given in water for 28 days to induce the kidney stone. For all in vivo activity model two doses of EAFGF (400 and 800 mg/kg) were given orally. After completion of study, body and kidney weight of rats were determined; various biochemical parameters including oxalate, calcium, urea, creatinine, uric acid, phosphate, magnesium and citrate levels in urine, serum and kidney homogenate using standard kits were measured; examination of histology of kidney for Calcium oxalate deposition was performed. Cell damage in cell tissue and urinary crystal size were also measured at the end of the study. The results were compared with a widely used herbal supplement, Cystone® in treatment of urolithiasis.
Results: EAFGF exhibited a concentration dependent inhibitory activity on calcium oxalate crystals in the in vitro study. Crystal nucleation (84.82 ± 0.52 % inhibition at 1500 µg/m); crystal aggregation (67.43±0.33 % inhibition at 1750 µg/ml); crystal growth (97.71±0.57 % inhibition at 2000 µg/ml); decreased number of calcium oxalate crystals produced in calcium oxalate solutions, and increase crystal dissolution (90.03±0.37 % at 2000 µg/ml) resulted in the in vitro study.
MDCK Cell line study showed that oxalate reduced the cell viability and increased LDH. In presence of EAFGF cell viability was increased (15.51±0.69 % to 77.35±0.50 % viability at 50 µg/ml) and reduced the LDH release level (87.69±0.59 % at 50 µg/ml). EAFGF showed concentration depended diuretics activity (8.51 ± 0.26 to 16.43±0.15 ml/100 gm/6 hr). Co-administration of EAFGF with sodium oxalate and ethylene glycol has significantly (p < 0.001) increased the urine volume and the level of calculus inhibitors (magnesium and citrate) and decreased the level of calculus promoters (Calcium, oxalate, phosphate, uric acid, urea) (Table 1). Histopathology of kidney showed significant improvement after treatment with EAFGF in both models (Fig 1). Urinary crystal size and cell damage count were decreased in EAFGF treated groups. HPTLC study indicated that EAFGF contains quercetin, rutin, gallic acid and catechin, which were quantified using validated HPLC method (Fig 2).
Conclusion: These results indicate the presence of quercetin and rutin in EAFGF, possessing significant activity in urolithiasis. It was confirmed by its diuretic activity, crystal nucleation, crystal growth, crystal aggregation, inhibitory effects and increased the crystal dissolution, ability to increase the levels of inhibitors (magnesium and citrate) and decrease the level of promoters (oxalate, calcium, urea, phosphate, uric acid) of urolithiasis. However, further studies are needed to isolate and characterize anti-urolithiatic compounds in their pure forms. The study also deserves toxicological evaluation and clinical trials to develop novel herbal drug for urolithiasis.
.jpg) Fig 1. HPLC chromatogram - EAFGF compounds - Rutin & Quercetin at 254 nm and Gallic acid & Catechin at 278 nm
Fig 1. HPLC chromatogram - EAFGF compounds - Rutin & Quercetin at 254 nm and Gallic acid & Catechin at 278 nm
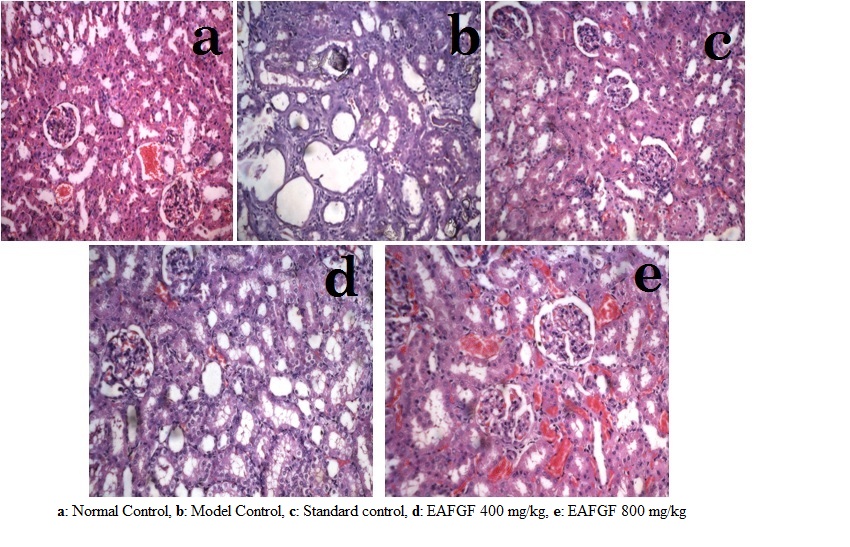 Fig 2. Histology of rat kidney - sodium oxalate model
Fig 2. Histology of rat kidney - sodium oxalate model
.jpg) Table 1. Urine, Serum & Kidney homogenate parameters - sodium oxalate model
Table 1. Urine, Serum & Kidney homogenate parameters - sodium oxalate model
Methods: For in vitro study, calcium oxalate crystals were prepared using the fresh solution of sodium oxalate and calcium chloride. Crystal nucleation and crystal growth assay were detected in UV spectrometry at 620 nm and 214 nm, respectively. Crystal aggregation assay was measured using turbidity meter. In a small test tube calcium oxalate crystal weight was measured before and after completion of study, to determine its dissolution. All study performed at varying ranges of EAFGF (200 to 2000 µg/ml). Cell viability test and Lactate Dehydrogenase Level (LDH) release after exposure of oxalate with or without EAFGF (concentration range 0.05 to 50 µg/ml) performed in MDCK Cell line. The identification and quantification of quercetin, rutin, catechin, and gallic acid, in EAFGF extract was performed using HPTLC and validated HPLC method, respectively.
For In vivo study, diuretic activity of EAFGF was performed using diuretic model in wistar albino rats. Sodium oxalate model and ethylene glycol models were used for urolithitic activity. 70 mg/kg sodium oxalate was given intraperitoneally for 7 days to induce the kidney stone in rats. 0.75 % v/v Ethylene Glycol was given in water for 28 days to induce the kidney stone. For all in vivo activity model two doses of EAFGF (400 and 800 mg/kg) were given orally. After completion of study, body and kidney weight of rats were determined; various biochemical parameters including oxalate, calcium, urea, creatinine, uric acid, phosphate, magnesium and citrate levels in urine, serum and kidney homogenate using standard kits were measured; examination of histology of kidney for Calcium oxalate deposition was performed. Cell damage in cell tissue and urinary crystal size were also measured at the end of the study. The results were compared with a widely used herbal supplement, Cystone® in treatment of urolithiasis.
Results: EAFGF exhibited a concentration dependent inhibitory activity on calcium oxalate crystals in the in vitro study. Crystal nucleation (84.82 ± 0.52 % inhibition at 1500 µg/m); crystal aggregation (67.43±0.33 % inhibition at 1750 µg/ml); crystal growth (97.71±0.57 % inhibition at 2000 µg/ml); decreased number of calcium oxalate crystals produced in calcium oxalate solutions, and increase crystal dissolution (90.03±0.37 % at 2000 µg/ml) resulted in the in vitro study.
MDCK Cell line study showed that oxalate reduced the cell viability and increased LDH. In presence of EAFGF cell viability was increased (15.51±0.69 % to 77.35±0.50 % viability at 50 µg/ml) and reduced the LDH release level (87.69±0.59 % at 50 µg/ml). EAFGF showed concentration depended diuretics activity (8.51 ± 0.26 to 16.43±0.15 ml/100 gm/6 hr). Co-administration of EAFGF with sodium oxalate and ethylene glycol has significantly (p < 0.001) increased the urine volume and the level of calculus inhibitors (magnesium and citrate) and decreased the level of calculus promoters (Calcium, oxalate, phosphate, uric acid, urea) (Table 1). Histopathology of kidney showed significant improvement after treatment with EAFGF in both models (Fig 1). Urinary crystal size and cell damage count were decreased in EAFGF treated groups. HPTLC study indicated that EAFGF contains quercetin, rutin, gallic acid and catechin, which were quantified using validated HPLC method (Fig 2).
Conclusion: These results indicate the presence of quercetin and rutin in EAFGF, possessing significant activity in urolithiasis. It was confirmed by its diuretic activity, crystal nucleation, crystal growth, crystal aggregation, inhibitory effects and increased the crystal dissolution, ability to increase the levels of inhibitors (magnesium and citrate) and decrease the level of promoters (oxalate, calcium, urea, phosphate, uric acid) of urolithiasis. However, further studies are needed to isolate and characterize anti-urolithiatic compounds in their pure forms. The study also deserves toxicological evaluation and clinical trials to develop novel herbal drug for urolithiasis.
.jpg) Fig 1. HPLC chromatogram - EAFGF compounds - Rutin & Quercetin at 254 nm and Gallic acid & Catechin at 278 nm
Fig 1. HPLC chromatogram - EAFGF compounds - Rutin & Quercetin at 254 nm and Gallic acid & Catechin at 278 nm Fig 2. Histology of rat kidney - sodium oxalate model
Fig 2. Histology of rat kidney - sodium oxalate model.jpg) Table 1. Urine, Serum & Kidney homogenate parameters - sodium oxalate model
Table 1. Urine, Serum & Kidney homogenate parameters - sodium oxalate model
Purpose: Urolithiasis is the most widespread urinary system disease that suffered globally. It formed due to deposition of acid salt in concentrated urine, which can cause infection, blockage, and damage to tissue of urinary system. Although the various conventional therapies are available for the urolithiasis, but side effects of the drugs cannot be avoided, application of extracorporeal lithotripsy, percutaneous nephrolithotomy and other minimally invasive techniques have made the treatment of urolithiasis more efficient in reducing their morbidity. In India, many herbal formulations are in use for the treatment of urolithiasis, known for its unquestionable efficacy and safety. Roots of Grewia flavescens used for treatment of urolithiasis. However, till date there is no scientific evaluation report of the above plant to be effective in treating urolithiasis. So, intends of present study to establish scientific rationality of Ethyl Acetate Fraction of Grewia flavescens (EAFGF) roots using in vitro study performed including nucleation assay, Calcium oxalate crystal growth and crystal aggregation assay, crystal dissolution, and Madin Darby Canine Kidney (MDCK) cell line study, while in vivo study performed using sodium oxalate induced urolithiasis model for preventive study and ethylene glycol induced model for curative study.
Methods: For in vitro study, calcium oxalate crystals were prepared using the fresh solution of sodium oxalate and calcium chloride. Crystal nucleation and crystal growth assay were detected in UV spectrometry at 620 nm and 214 nm, respectively. Crystal aggregation assay was measured using turbidity meter. In a small test tube calcium oxalate crystal weight was measured before and after completion of study, to determine its dissolution. All study performed at varying ranges of EAFGF (200 to 2000 µg/ml). Cell viability test and Lactate Dehydrogenase Level (LDH) release after exposure of oxalate with or without EAFGF (concentration range 0.05 to 50 µg/ml) performed in MDCK Cell line. The identification and quantification of quercetin, rutin, catechin, and gallic acid, in EAFGF extract was performed using HPTLC and validated HPLC method, respectively.
For In vivo study, diuretic activity of EAFGF was performed using diuretic model in wistar albino rats. Sodium oxalate model and ethylene glycol models were used for urolithitic activity. 70 mg/kg sodium oxalate was given intraperitoneally for 7 days to induce the kidney stone in rats. 0.75 % v/v Ethylene Glycol was given in water for 28 days to induce the kidney stone. For all in vivo activity model two doses of EAFGF (400 and 800 mg/kg) were given orally. After completion of study, body and kidney weight of rats were determined; various biochemical parameters including oxalate, calcium, urea, creatinine, uric acid, phosphate, magnesium and citrate levels in urine, serum and kidney homogenate using standard kits were measured; examination of histology of kidney for Calcium oxalate deposition was performed. Cell damage in cell tissue and urinary crystal size were also measured at the end of the study. The results were compared with a widely used herbal supplement, Cystone® in treatment of urolithiasis.
Results: EAFGF exhibited a concentration dependent inhibitory activity on calcium oxalate crystals in the in vitro study. Crystal nucleation (84.82 ± 0.52 % inhibition at 1500 µg/m); crystal aggregation (67.43±0.33 % inhibition at 1750 µg/ml); crystal growth (97.71±0.57 % inhibition at 2000 µg/ml); decreased number of calcium oxalate crystals produced in calcium oxalate solutions, and increase crystal dissolution (90.03±0.37 % at 2000 µg/ml) resulted in the in vitro study.
MDCK Cell line study showed that oxalate reduced the cell viability and increased LDH. In presence of EAFGF cell viability was increased (15.51±0.69 % to 77.35±0.50 % viability at 50 µg/ml) and reduced the LDH release level (87.69±0.59 % at 50 µg/ml). EAFGF showed concentration depended diuretics activity (8.51 ± 0.26 to 16.43±0.15 ml/100 gm/6 hr). Co-administration of EAFGF with sodium oxalate and ethylene glycol has significantly (p < 0.001) increased the urine volume and the level of calculus inhibitors (magnesium and citrate) and decreased the level of calculus promoters (Calcium, oxalate, phosphate, uric acid, urea) (Table 1). Histopathology of kidney showed significant improvement after treatment with EAFGF in both models (Fig 1). Urinary crystal size and cell damage count were decreased in EAFGF treated groups. HPTLC study indicated that EAFGF contains quercetin, rutin, gallic acid and catechin, which were quantified using validated HPLC method (Fig 2).
Conclusion: These results indicate the presence of quercetin and rutin in EAFGF, possessing significant activity in urolithiasis. It was confirmed by its diuretic activity, crystal nucleation, crystal growth, crystal aggregation, inhibitory effects and increased the crystal dissolution, ability to increase the levels of inhibitors (magnesium and citrate) and decrease the level of promoters (oxalate, calcium, urea, phosphate, uric acid) of urolithiasis. However, further studies are needed to isolate and characterize anti-urolithiatic compounds in their pure forms. The study also deserves toxicological evaluation and clinical trials to develop novel herbal drug for urolithiasis.
.jpg) Fig 1. HPLC chromatogram - EAFGF compounds - Rutin & Quercetin at 254 nm and Gallic acid & Catechin at 278 nm
Fig 1. HPLC chromatogram - EAFGF compounds - Rutin & Quercetin at 254 nm and Gallic acid & Catechin at 278 nm
 Fig 2. Histology of rat kidney - sodium oxalate model
Fig 2. Histology of rat kidney - sodium oxalate model
.jpg) Table 1. Urine, Serum & Kidney homogenate parameters - sodium oxalate model
Table 1. Urine, Serum & Kidney homogenate parameters - sodium oxalate model
Methods: For in vitro study, calcium oxalate crystals were prepared using the fresh solution of sodium oxalate and calcium chloride. Crystal nucleation and crystal growth assay were detected in UV spectrometry at 620 nm and 214 nm, respectively. Crystal aggregation assay was measured using turbidity meter. In a small test tube calcium oxalate crystal weight was measured before and after completion of study, to determine its dissolution. All study performed at varying ranges of EAFGF (200 to 2000 µg/ml). Cell viability test and Lactate Dehydrogenase Level (LDH) release after exposure of oxalate with or without EAFGF (concentration range 0.05 to 50 µg/ml) performed in MDCK Cell line. The identification and quantification of quercetin, rutin, catechin, and gallic acid, in EAFGF extract was performed using HPTLC and validated HPLC method, respectively.
For In vivo study, diuretic activity of EAFGF was performed using diuretic model in wistar albino rats. Sodium oxalate model and ethylene glycol models were used for urolithitic activity. 70 mg/kg sodium oxalate was given intraperitoneally for 7 days to induce the kidney stone in rats. 0.75 % v/v Ethylene Glycol was given in water for 28 days to induce the kidney stone. For all in vivo activity model two doses of EAFGF (400 and 800 mg/kg) were given orally. After completion of study, body and kidney weight of rats were determined; various biochemical parameters including oxalate, calcium, urea, creatinine, uric acid, phosphate, magnesium and citrate levels in urine, serum and kidney homogenate using standard kits were measured; examination of histology of kidney for Calcium oxalate deposition was performed. Cell damage in cell tissue and urinary crystal size were also measured at the end of the study. The results were compared with a widely used herbal supplement, Cystone® in treatment of urolithiasis.
Results: EAFGF exhibited a concentration dependent inhibitory activity on calcium oxalate crystals in the in vitro study. Crystal nucleation (84.82 ± 0.52 % inhibition at 1500 µg/m); crystal aggregation (67.43±0.33 % inhibition at 1750 µg/ml); crystal growth (97.71±0.57 % inhibition at 2000 µg/ml); decreased number of calcium oxalate crystals produced in calcium oxalate solutions, and increase crystal dissolution (90.03±0.37 % at 2000 µg/ml) resulted in the in vitro study.
MDCK Cell line study showed that oxalate reduced the cell viability and increased LDH. In presence of EAFGF cell viability was increased (15.51±0.69 % to 77.35±0.50 % viability at 50 µg/ml) and reduced the LDH release level (87.69±0.59 % at 50 µg/ml). EAFGF showed concentration depended diuretics activity (8.51 ± 0.26 to 16.43±0.15 ml/100 gm/6 hr). Co-administration of EAFGF with sodium oxalate and ethylene glycol has significantly (p < 0.001) increased the urine volume and the level of calculus inhibitors (magnesium and citrate) and decreased the level of calculus promoters (Calcium, oxalate, phosphate, uric acid, urea) (Table 1). Histopathology of kidney showed significant improvement after treatment with EAFGF in both models (Fig 1). Urinary crystal size and cell damage count were decreased in EAFGF treated groups. HPTLC study indicated that EAFGF contains quercetin, rutin, gallic acid and catechin, which were quantified using validated HPLC method (Fig 2).
Conclusion: These results indicate the presence of quercetin and rutin in EAFGF, possessing significant activity in urolithiasis. It was confirmed by its diuretic activity, crystal nucleation, crystal growth, crystal aggregation, inhibitory effects and increased the crystal dissolution, ability to increase the levels of inhibitors (magnesium and citrate) and decrease the level of promoters (oxalate, calcium, urea, phosphate, uric acid) of urolithiasis. However, further studies are needed to isolate and characterize anti-urolithiatic compounds in their pure forms. The study also deserves toxicological evaluation and clinical trials to develop novel herbal drug for urolithiasis.
.jpg) Fig 1. HPLC chromatogram - EAFGF compounds - Rutin & Quercetin at 254 nm and Gallic acid & Catechin at 278 nm
Fig 1. HPLC chromatogram - EAFGF compounds - Rutin & Quercetin at 254 nm and Gallic acid & Catechin at 278 nm Fig 2. Histology of rat kidney - sodium oxalate model
Fig 2. Histology of rat kidney - sodium oxalate model.jpg) Table 1. Urine, Serum & Kidney homogenate parameters - sodium oxalate model
Table 1. Urine, Serum & Kidney homogenate parameters - sodium oxalate model
Code of conduct/disclaimer available in General Terms & Conditions
{{ help_message }}
{{filter}}